
Insights+: EMA Marketing Authorization of New Drugs in April 2024
Shots:
-
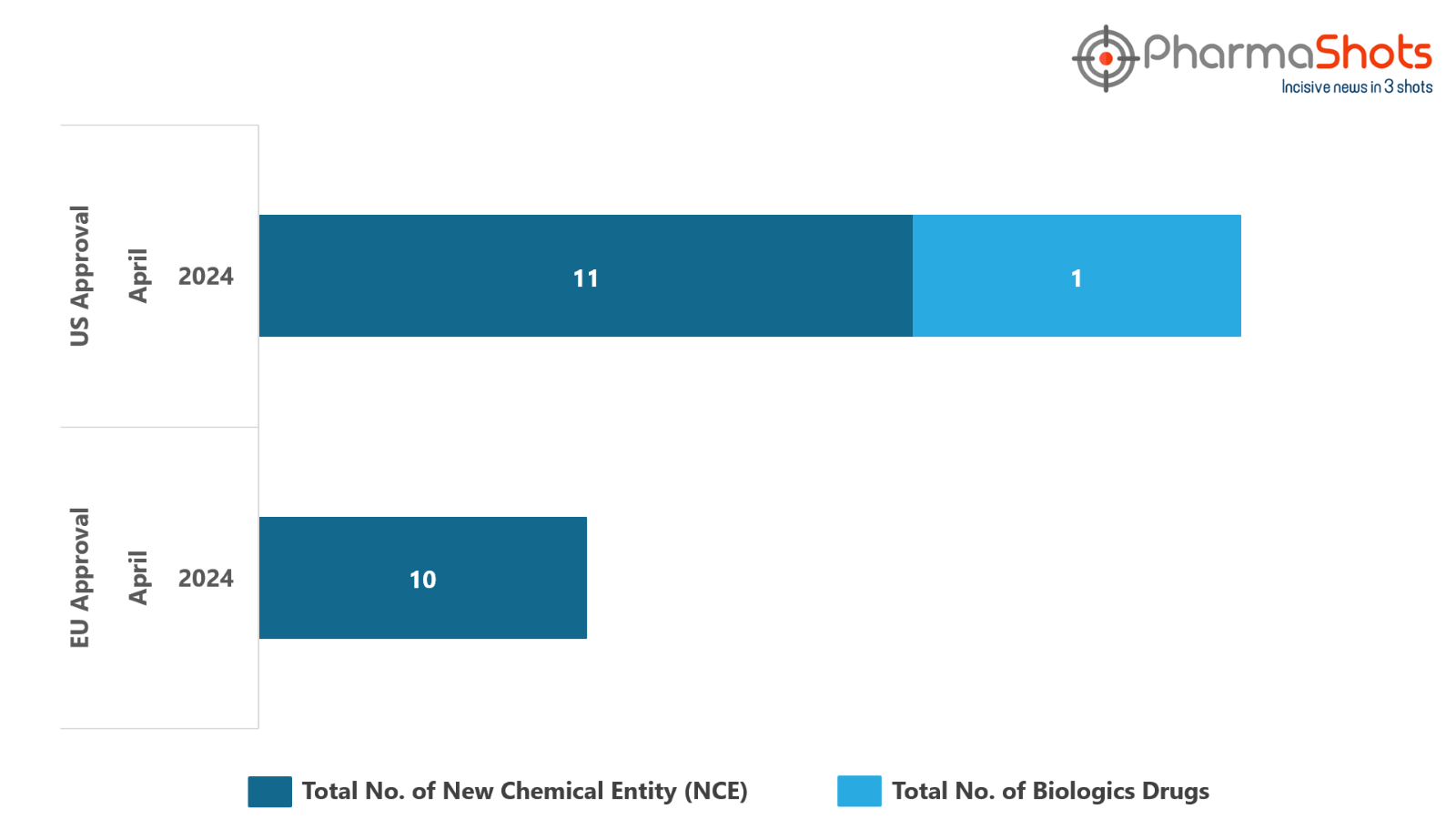
The EC approved 10 New Chemical Entities in April 2024, leading to treatments for patients and advances in the healthcare industry
-
In April 2024, the major highlighted drugs were Takeda’s Fruzaqla to treat Adults with mCRC and Johnson & Johnson’s Rybrevant for the treatment NSCLC
-
PharmaShots has compiled a list of 5 drugs that have received positive opinion by the EMA’s CHMP
Product Name: Fruzaqla
Active ingredient: Fruquintinib
Company: Takeda
Date: Apr 26, 2024
Disease: Metastatic Colorectal Cancer
Shots:
-
The CHMP has granted positive opinion to the company’s fruquintinib (VEGFR-1, -2 & -3 inhibitor) for previously treated metastatic colorectal cancer (mCRC) adults. Takeda holds its exclusive global rights outside of mainland China, Hong Kong & Macau while HUTCHMED has China rights
-
The opinion was based on P-III (FRESCO-2) study assessing fruquintinib + best supportive care (BSC) vs PBO + BSC in previously treated mCRC patients
-
The study met both 1EPs and 2EPs demonstrating improved OS & PFS with consistent safety profile irrespective of previous studies. The data was published in The Lancet
Product Name: Rybrevant
Active ingredient: Amivantamab
Company: Janssen-Cilag International (Johnson & Johnson)
Date: Apr 26, 2024
Disease: NSCLC
Shots:
-
The company's Rybrevant + CT (carboplatin & pemetrexed) has gained the CHMP’s positive opinion as a 1L therapy for NSCLC associated with activating EGFR exon 20 insertion mutations
-
The opinion was based on the P-III (PAPILLON) trial assessing the safety & efficacy of Rybrevant + CT vs CT in NSCLC patients (n=308) with EGFR exon 20 insertion mutations that showed improved PFS (1EP) & a trend favoring interim OS. 7% TRAEs leading to discontinuation
-
Rybrevant is a bispecific Ab targeting activated & resistant EGFR mutation as well as MET mutations and amplifications
Product Name: Opdivo
Active ingredient: Nivolumab
Company: Bristol Myers Squibb
Date: Apr 26, 2024
Disease: Urothelial Carcinoma
Shots:
-
The CHMP has granted positive opinion to Opdivo + CT (cisplatin & gemcitabine) as 1L treatment of metastatic or unresectable urothelial carcinoma adults (n=608). The decision is anticipated in Jun 2024
-
The opinion was based on a sub-study of the P-III (CheckMate–901), assessing Opdivo (360mg, Q3W, 6 cycles) + CT (cisplatin & gemcitabine) followed by Opdivo (480mg, Q4W) vs SoC CT alone to treat urothelial cancer (n=608)
-
The study showed improved OS & PFS. The combination reduced the death risk by 22% with a mOS of 21.7mos. vs 18.9mos. (CT alone) at a median follow-up of 33mos. and reduced the risk of disease progression or death by 28%, with a mPFS of 7.9mos. vs 7.6mos. (CT alone)
Product Name: Sirturo
Active ingredient: Bedaquiline
Company: Johnson & Johnson
Date: Apr 29, 2024
Disease: Tuberculosis
Shots:
-
The CHMP has granted positive opinion to the company’s Sirturo (bedaquiline) and recommended approving its Type II variation plus transitioning its conditional marketing authorization into a standard marketing authorization
-
The opinion was based on the data from P-III (STREAM) stage 2 trial assessing the safety & efficacy of bedaquiline-containing regimen to treat MDR-TB, depicting improvement and confirming its positive risk-benefit
-
Additionally, Sirturo received the US FDA’s accelerated approval in Dec 2012 & the EMA’s conditional approval in Mar 2014 based on the P-II study results. Its sNDA, submitted to the US FDA in Aug 2023, is underway for full approval
5. AstraZeneca’s Truqap Plus Faslodex Receives the CHMP’s Positive Opinion for Treating Breast Cancer
Product Name: Truqap + Faslodex
Active ingredient: Capivasertib + fulvestrant
Company: AstraZeneca
Date: Apr 29, 2024
Disease: Breast Cancer
Shots:
-
The CHMP's positive opinion was based on the P-III (CAPItello-291) study assessing Truqap + Faslodex vs PBO + Faslodex to treat ER+, HER2‑ locally advanced or metastatic breast cancer patients (n=708) having ≥1 PIK3CA, AKT1 or PTEN-alterations after recurrence or progression on or after an endocrine-based regimen
-
The study demonstrated reduction in the disease progression & death risk by 50% in patients having PI3K, AKT or PTEN alterations with mPFS of 7.3mos. vs 3.1mos. The data was published in The New England Journal of Medicine
-
Regulatory applications are under review across China & other countries. Based on P-III results, combination for same indication has been approved in the US, Japan & other countries
Note:
According to the EMA’s April 2024 approval list, the following drugs were also approved; however, no PR was available:
-
Reyataz
-
Triumeq
-
Rozlytrek
Following drugs received CHMP’s Opinion; however, no PR was available:
-
Obgemsa
-
Eribulin Baxter
Related Post: Insights+: EMA Marketing Authorization of New Drugs in March 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














